Magnetic resonance imaging (MRI) detected lumbar pathology may be a significant factor in the context of low back pain (LBP) recurrence (M Hildebrandt et al, 2017). Investigating lumbar pathology with MRI, a non-invasive technique is a standard practice in medicine (Milette et al, 1999). Since MRI imaging reveals anatomical and morphologic features of the spine, the results do not directly determine the cause of pain. The lack of a consistent association between the underlying pathology and the observed signs and symptoms is a major challenge to the use of spinal imaging techniques, such as MRI (Hamanishi et al 2004, Beattie et al 1994). In most cases, images obtained from MRI scans have little value in identifying symptomatically significant pathology and therefore researchers suggest that the findings from MRI scans should not be used for prognosis or treatment planning outside of the context of the patient’s clinical presentation (Milette et al 1999, Hamanishi et al 2004). However, recent evidence suggests that physical therapy treatment for discogenic lumbar disorders can be directed by the size and shape of the disc in an MRI scan.
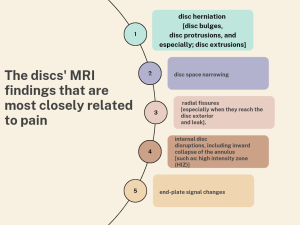
MRI images can help us identify disc herniation (Jensen et al 2008), disc narrowing (Jensen et al 1994, Videman et al 2003), radial fissures (Jensen et al 1994, Hassett et al 2003), disc leak (Moneta et al 1994), and internal disc disruptions, which includes the tear of the annulus (Videman and Nurminen 2004). A high-intensity zone (HIZ) on T2-weighted images of the posterior annulus has been shown to have excellent specificity in identifying discogenic pathology (Schwarzer et al 1995, Aprill and Bogduk 1992), and other painful pathologies such as growth-plate fractures, Schmorl’s nodes (Beattie et al 1994), Modic (type II) changes (Jensen et al 2008) and disc bulging (Beattie Et al 1994, Jensen et al 2008, Jensen et al 1994, Videman et al 2003, Boos et al 1995). However, disc signal intensity on MRI has a limited relationship to pain (Videman et al 2003). On the other hand, research shows that the degree of disc and facet joint degeneration has a positive association with excessive translational motion when dynamic MRI is used to evaluate motion in a standing position (Min Ho Kong et al.2009, J Bräm et al. 1998), while the degree of facet joint degeneration has a negative association with excessive angular motion. Furthermore, segmental instability (greater than 3 mm translation) on X-ray has also been linked to other spine diseases, such as annular rips (HIZ) or traction spurs identified using MRI scans. When compared to lower grades of disc degeneration, there is lesser angular and translational motion with severe disc degeneration (grade V). Also, despite disagreements about fatty infiltration of lumbar multifidi and the presence of pain, there is some evidence of this relationship and its relevance to segmental instability (Per Kjaer et al. 2007).
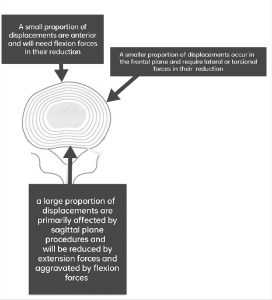
Loading in the favored (preferred) direction of movement or optimal spinal position lessens neural impingement, decreases neural irritation and edema, which in turn leads to centralization of the pain, lessens signs and symptoms, improves the decreased variety of movement (lengthy et al 2004) and predicts good treatment effects (Werneke et al 2011, lengthy et al 2004, Skytte Et al 2005). The reduction of symptoms is in particular associated with the neural decompression (Abdulwahab and Sabbahi, 2000; Mazzocchio, 2001) of sensory and motor nerves. But, loading in the undesired direction of movement or spinal position worsens or peripheralizes the pain (McKenzie and can 2003) and makes the movement difficult to carry out (Riddle et al 1993). A dynamic disc model has been hypothesized as the underlying mechanism for these phenomena and may be explained by the adjustments in disc displacement (Kolber and Hanney 2009). Clinical findings reveal that a significant percentage of displacements are mostly influenced by sagittal plane treatments and will be exacerbated by flexion forces as opposed to being reduced by them. Lesser amounts of displacements take place in the frontal plane and must be reduced by lateral or torsional forces. Only a small part of displacements are anterior, and their correction requires flexion forces (McKenzie 1981). It is well-known that static extension in lying (EIL) is advocated for acute posterior disc bulges with 70% of the disc height (McGill, 2016). Also, in patients with L4-5 lumber disc prolapse of much less than 3 mm have to be placed in extension at the same time as those of more than 4 mm should be placed in axial rotation and kept away from extension based on the finite model (Aly MM, et al. 2015). In this context, researchers report that pain centralization (i.e., recovery or reduction of pain) is associated with MRI findings of lumbar disc abnormality (Laslett M, et al. 2011). Yoon-Ho Kim et al. 2007 found that when the internal disc is disrupted, cervical extension causes the nucleus pulposus to migrate anteriorly and away from the posterior disc margin, which may have a clinical impact on discogenic neck pain. So, based on clinical and mechanical responses to loading techniques, discogenic and/or radicular syndrome is managed (McKenzie 1981), which in turn correlates well with various imaging modalities like MRI and discography.
MRI and leg pain have a good correlation:
In a study done by Pople and Griffith, 1994, the authors found that a disc protrusion was present in 96% of persons who had sciatica but no back pain.
MRI and neurological deficits have a good correlation:
A study by NV, et al. 2019 reported that non-contained discs and pre-existing spinal canal stenosis are two possible radiological risk factors for developing neurological deficits. Also, a study done by Nordberg CL, et al. 2021 found that standing position revealed higher levels of nerve root compression for paracentral herniated discs. Furthermore, in a study conducted by Henrietta N Redebrandt et al. 2022, the authors found that in patients with cervical radiculopathy, the clinical-neurological evaluation identified the same cervical root in 31% of cases or a cervical root close to the most affected cervical root on MRI in 28% of cases, which concluded that in 59% of the patients, the clinical and MRI findings were in agreement.
A change in pain is linked to a change in herniation size:
Seo et al, 2016 reported that in the group with lesser herniations, pain relief occurred more immediately. Fagerlund et al, 1990 found a substantial favorable link between the reduction in the size of the particular hernia and the recovery from sciatic pain, and Kesikburun et al. 2019, also found that with conservative treatment, the majority of patients with extruded lumbar disc herniation may see improvement in symptoms and function over the long term as well as a reduction in the size of the herniated disc. So, a change in discomfort is connected to a change in disc size. The fact that a herniation’s size is unrelated to pain but its growth over time is somewhat incongruous. Again, it appears to be clear in the cervical spine, as numerous investigations (J. Y. Maigne et al. 1994; K. Mochida et al. 1998) discovered a relationship between the presence or lack of symptoms and the presence or absence of disc protrusions seen on imaging.
Pathological MRI features in asymptomatic individuals:
Wilberger Jr et al. 1983, conducted a study about asymptomatic patients’ incidental MRI abnormalities and their clinical significance. Asymptomatic prolapsed or herniated discs, as represented by incidental lumbar MRI findings, in individuals who did not have any symptoms at the time of MRI scans had a higher likelihood of developing symptoms in the future. The authors reported that an MRI finding known as ‘spondylolisthesis’ suggested that people without baseline pain would experience more severe pain in the future, whereas people with baseline pain would experience less severe future pain. Multiple MRI findings (3 or more pathological features) were linked to moderately increased future pain among people without LBP at baseline. So, lumbar sciatica may occur later in these patients with asymptomatic aberrant MRI lumbar results. Patients who have incidental lumbar imaging anomalies should be properly monitored and given the proper care.
How can we use MRI findings to select patients for conservative management and surgery?
In 2020, Lawrence Walter Mysliwiec, et al. created a classification for MRI lumbar disc morphology that categorizes herniation size as simple as 1-2-3 and location as A-B-C, with inter-examiner reliability of 98%. This classification may predict the best criteria for patient selection for surgery in those with pathological spinal MRI features. It was discovered that types 2-B and 2-AB of herniation were most frequently selected for surgery in each group, indicating the relevance of both size and location. JR Wilson, 2013 also related the occurrence, timing, and factors that influence the development of myelopathy in asymptomatic patients with cervical stenosis brought on by spondylosis or ossification of the posterior longitudinal ligament, and recommended that patients who presented with clinical or electrophysiological evidence of cervical radicular dysfunction or central conduction deficits and those who had cervical canal stenosis and cord compression secondary to spondylosis appeared to be at higher risk for developing myelopathy and therefore should be advised to consider surgical treatment.
Key takeaway:
Findings from imaging modalities such as MRI scans should be used as part of the clinical reasoning process to inform and guide treatment decisions.
References:
-
Abdulwahab, S.S., Sabbahi, M., 2000. Neck retractions, cervical root decompression, and radicular pain. J. Orthop. Sports Phys. Ther. 30 (1), 4–12.
-
Aprill C, Bogduk N (1992). High-intensity zone: a diagnostic sign of painful disc on magnetic resonance imaging. Br J Radiol;65:361-69.
-
Beattie PF, Brooks WM, Rothstein JM, Sibbitt WL et al (1994). Effect of lordosis on the position of the nucleus pulposus in supine subjects. A study using magnetic resonance imaging. Spine;19:2096-102.
-
Boos N, Rieder R, Schade V et al (1995). Volvo award in clinical sciences. The diagnostic accuracy of magnetic resonance imaging, work perception, and psychosocial factors in identifying symptomatic disc herniations. Spine;20:2613-25. Pain centralization and lumbar disc MRI findings in chronic low back pain patients.
-
Bram J, Zanetti M, Min K, Hodler J. MR abnormalities of the intervertebral disks and adjacent bone marrow as predictors of segmental instability of the lumbar spine. Acta Radiol 1998;39: 18–23.
-
Fagerlund MK, Thelander U, Friberg S. Size of lumbar disc hernias measured using computed tomography and related to sciatic symptoms. Acta Radiol. 1990 Nov;31(6):555-8. PMID: 2278776.
-
Hamanishi C, Kawabata T, Yosii T et al (2004). Schmorl’s nodes on magnetic resonance imaging. Their incidence and clinical relevance. Spine;19:450-3.
-
Hassett G, Hart DJ, Manek NJ et al (2003). Risk factors for progression of lumbar spine disc degeneration: The Chingford Study. Arthr Rheum;48:3112-7.
-
Hildebrandt M, Fankhauser G, Meichtry A, Luomajoki H. Correlation between lumbar dysfunction and fat infiltration in lumbar multifidus muscles in patients with low back pain. BMC Musculoskelet Disord. 2017 Jan 10;18(1):12. doi: 10.1186/s12891-016-1376-1. PMID: 28068962; PMCID: PMC5223418.
-
Jensen MC, Brant-Zawadzki MN, Obuchowski N et al (1994). Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med;331:69 -73.
-
Jensen TS, Karppinen J, Sorensen JS et al (2008). Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J;17:1407-22.
-
Kesikburun B, Eksioglu E, Turan A, Adiguzel E, Kesikburun S, Cakci A. Spontaneous regression of extruded lumbar disc herniation: Correlation with clinical outcome. Pak J Med Sci. 2019 Jul-Aug;35(4):974-980. doi: 10.12669/pjms.35.4.346. PMID: 31372127; PMCID: PMC6659070.
-
Kilpikoski S, PT, PhD, Laslett M, FNZCP, PhD, Airaksinen O, MD, DMedSci, Kankaanpää M, MD, DMedSci, Alen M, MD, DMedSci (FIN) Reprinted with kind permission from Manuelle Therapie (June 2011).
-
Kim YH, Kim SI, Park S, Hong SH, Chung SG. Effects of Cervical Extension on Deformation of Intervertebral Disk and Migration of Nucleus Pulposus. PM R. 2017 Apr;9(4):329-338. doi: 10.1016/j.pmrj.2016.08.027. Epub 2016 Sep 6. PMID: 27613586.
-
Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007 Jan 25;5:2. doi: 10.1186/1741-7015-5-2. PMID: 17254322; PMCID: PMC1796893.
-
Kong MH, Hymanson HJ, Song KY, Chin DK, Cho YE, Yoon do H, et al. Kinetic magnetic resonance imaging analysis of abnormal segmental motion of the functional spine unit.J Neurosurg Spine 2009;10: 357–65.
-
Kolber MJ, Hanney WJ (2009). The dynamic disc model: a systematic review of the literature. Phys Ther Rev;14:181-295.
-
Long A, Donelson R, Fung T (2004). Does it matter which exercise? A randomized controlled trial of exercise for low back pain.Spine;29:2593-602.
-
Maigne JY, Deligne L. Computed tomographic follow-up study of 21 cases of nonoperatively treated cervical intervertebral soft disc herniation. Spine (Phila Pa 1976). 1994 Jan 15;19(2):189-91. doi: 10.1097/00007632-199401001-00013. PMID: 8153829.
-
Mazzocchio, R., 2001. Comment on Soleus H-reflex changes during loading and unloading of the spine and their relation to the diagnosis of lumbosacral radiculopathy in mechanical back pain. Clin. Neurophysiol. 112 (10), 1952– 1954.
-
McKenzie RA, May S (editors)(2003). The lumbar spine mechanical diagnosis and therapy. Waikanae: Spinal Publication Ltd, p. 355-65.
-
McKenzie RA (1981). The Lumbar Spine. Mechanical Diagnosis and Therapy. Spinal Publications, New Zealand.
-
Milette PC, Fontaine S, Lepanto L et al (1999). Differentiating lumbar disc protrusions, disc bulges and discs with normal contour but abnormal signal intensity. Magnetic resonance imaging with discographic correlations. Spine;1:44-53.
-
Mochida K, Komori H, Okawa A, Muneta T, Haro H, Shinomiya K. Regression of cervical disc herniation observed on magnetic resonance images. Spine (Phila Pa 1976). 1998 May 1;23(9):990-5; discussion 996-7. doi: 10.1097/00007632-199805010-00005. PMID: 9589536.
-
Moneta GB, Videman T, Kaivanto K et al (1994). Reported pain during lumbar discography as a function of anular ruptures and disc degeneration. A re-analysis of 833 discograms. Spine;19:1968-74.
-
Mysliwiec LW, Cholewicki J, Winkelpleck MD, Eis GP. MSU classification for herniated lumbar discs on MRI: toward developing objective criteria for surgical selection. Eur Spine J. 2010 Jul;19(7):1087-93. doi: 10.1007 s00586-009-1274-4. Epub 2010 Jan 19. PMID: 20084410; PMCID: PMC2900017.
-
Nordberg CL, Boesen M, Fournier GL, Bliddal H, Hansen P, Hansen BB. Positional changes in lumbar disc herniation during standing or lumbar extension: a cross-sectional weight-bearing MRI study. Eur Radiol. 2021 Feb;31(2):804-812. doi: 10.1007/s00330-020-07132-w. Epub 2020 Aug 21. PMID: 32822052.
-
Nv A, Rajasekaran S, Ks SVA, Kanna RM, Shetty AP. Factors that influence neurological deficit and recovery in lumbar disc prolapse-a narrative review. Int Orthop. 2019 Apr;43(4):947-955. doi: 10.1007/s00264-018-4242-y. Epub 2018 Nov 24. PMID: 30474689.
-
Redebrandt HN, Brandt C, Hawran S, Bendix T. Clinical evaluation versus magnetic resonance imaging findings in patients with radicular arm pain-A pragmatic study. Health Sci Rep. 2022 Apr 10;5(3):e589. doi: 10.1002/hsr2.589. PMID: 35434382; PMCID: PMC8995534.
-
Riddle DL, Rothstein JM (1993). Intertester reliability of McKenzie’s classifications of the syndrome types present in patients with low-back pain. Spine;18:1333- 44.
-
Seo JY, Roh YH, Kim YH, Ha KY. Three-dimensional analysis of volumetric changes in herniated discs of the lumbar spine: does spontaneous resorption of herniated discs always occur? Eur Spine J. 2016 May;25(5):1393-1402. doi: 10.1007/s00586-014-3587-1. Epub 2014 Sep 25. PMID: 25253299.
-
Pople IK, Griffith HB. Prediction of an extruded fragment in lumbar disc patients from clinical presentations. Spine (Phila Pa 1976). 1994 Jan 15;19(2):156-8. doi: 10.1097/00007632-199401001-00007. PMID: 8153823.
-
Schwarzer AC, Aprill CN, Derby R et al (1995). The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine;20:1878-83.
-
Skytte L. May S, Petersen P (2005). Centralization: It’s prognostic value in patients with referred symptoms and sciatica. Spine;30:E293-99.
-
Videman T, Battie MC, Gibbons LE et al (2003). Associations between back pain history and lumbar MRI findings. Spine;28:582-8.
-
Videman T, Nurminen M (2004). The occurrence of annular tear and their relation to lifetime back pain history: A cadaveric study using barium sulphate discography. Spine;29:2668-76.
-
Werneke M, Hart D, Cutrone G (2011). Association between directional preference and centralization phenomenon in patients with low back pain. JOSPT;41:22-31.
-
Wilberger JE Jr, Pang D. Syndrome of the incidental herniated lumbar disc. J Neurosurg. 1983 Jul;59(1):137-41. doi: 10.3171/jns.1983.59.1.0137. PMID: 6864267.
-
Wilson JR, Barry S, Fischer DJ, Skelly AC, Arnold PM, Riew KD, Shaffrey CI, Traynelis VC, Fehlings MG. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patient with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976). 2013 Oct 15;38(22 Suppl 1):S37-54. doi: 10.1097/BRS.0b013e3182a7f2e7. PMID: 23963005.